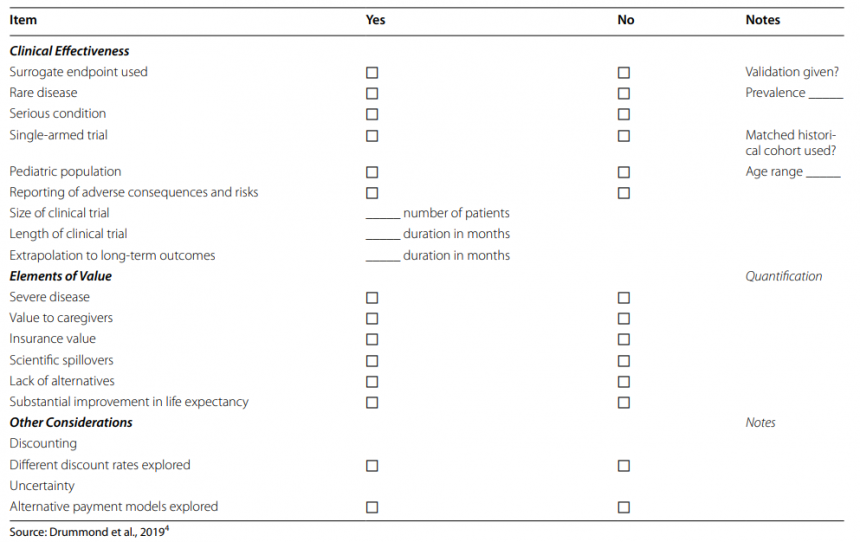
Drummond et al. (2023) explore how health technology assessment bodies approach value assessment for cell and gene therapies (also known as Advanced Therapy Medicinal Products) in Europe. They conducted a targeted literature review on clinical and economic evidence needs and analyzed HTA reports and managed entry agreements for 9 therapies in 10 indications across 8 major jurisdictions. Using a data extraction form from a previous study, they assessed the positive, negative, and neutral citations of key elements.

While some societal value elements were noted in ATMP value assessment, they were not consistently included. The paper provides valuable insights into this topic.


For more detailed insights, the full paper can be accessed here.
. If the provided articles seems to be less than 200 characters or it is an intro of the author, then try to generate an articles using this title.HTA and cell and gene therapy – Healthcare Economist