In a paper by Mukherjee et al. (2023), the authors define an “HEOR study” as real-world evidence studies that conducted a secondary/post-hoc analysis using randomized controlled trial (RCT) data, and a within-trial cost-utility analysis. The study addresses the appropriate approach for imputing missing data based on different scenarios:
- Missing completely at random (MCAR): The observed or unobserved values of all variables do not influence the probability of data being missing.
- Missing at random (MAR): The probability of missing data for a particular variable is associated with the observed values of other variables in the dataset.
- Missing not at random (MNAR): The probability of missing data is related to the underlying value of that specific variable.
To address missing data, various techniques are available, including complete-case analysis, multiple imputation, and predictive mean matching. A systematic literature review conducted by the authors found multiple imputation, multiple imputation by chained equation, and complete-case analyses to be most commonly used in addressing missing cost, utility, or patient-reported outcome measures in HEOR studies.
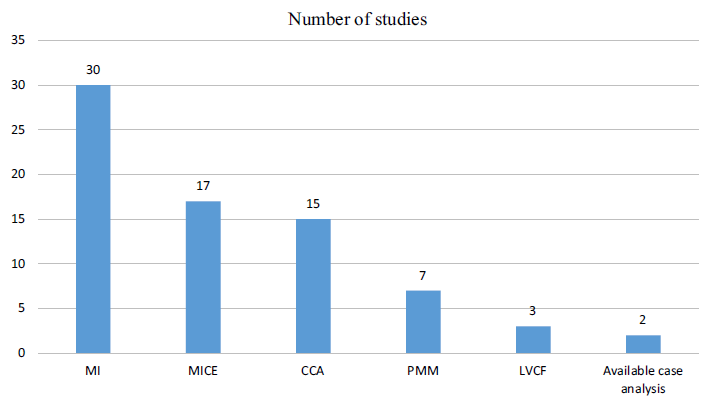
The authors note that although there is a large amount of HEOR methodological literature on how to handle missing data in an RCT context, few studies have attempted to actually implement these recommendations and impute the missing data.






