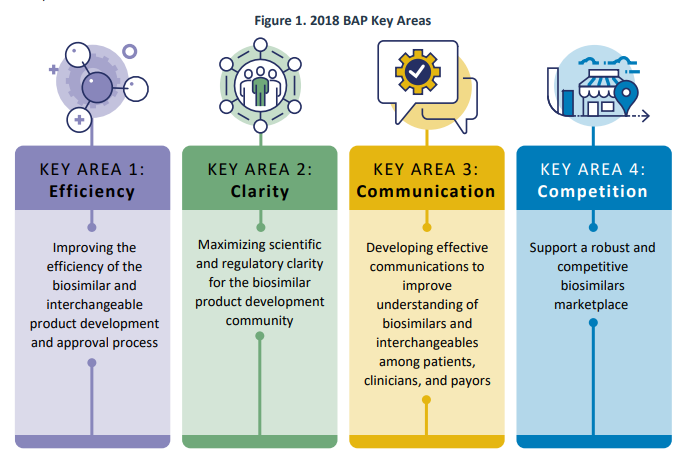
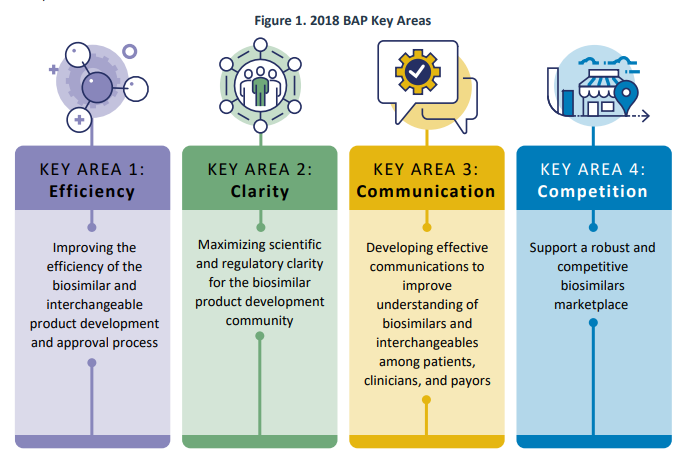
In July 2018, the FDA unveiled the Biosimilars Action Plan (BAP) with the goal of enhancing access to biosimilars for the American public. The BAP emphasized four key areas of focus:
A recent FDA report highlights the progress made since the BAP’s inception. Efforts have primarily centered on the development of guidance documents, increased staff, educational materials, public hearings, and regulatory updates. Additionally, the FDA has launched new data resources, such as an updated version of the Purple Book. Collaboration with agencies like the FTC has resulted in joint statements and workshops aimed at promoting a competitive marketplace for biosimilars. More details can be found in the full report.

Explore the key initiatives outlined by the FDA in support of the Biosimilars Action Plan.