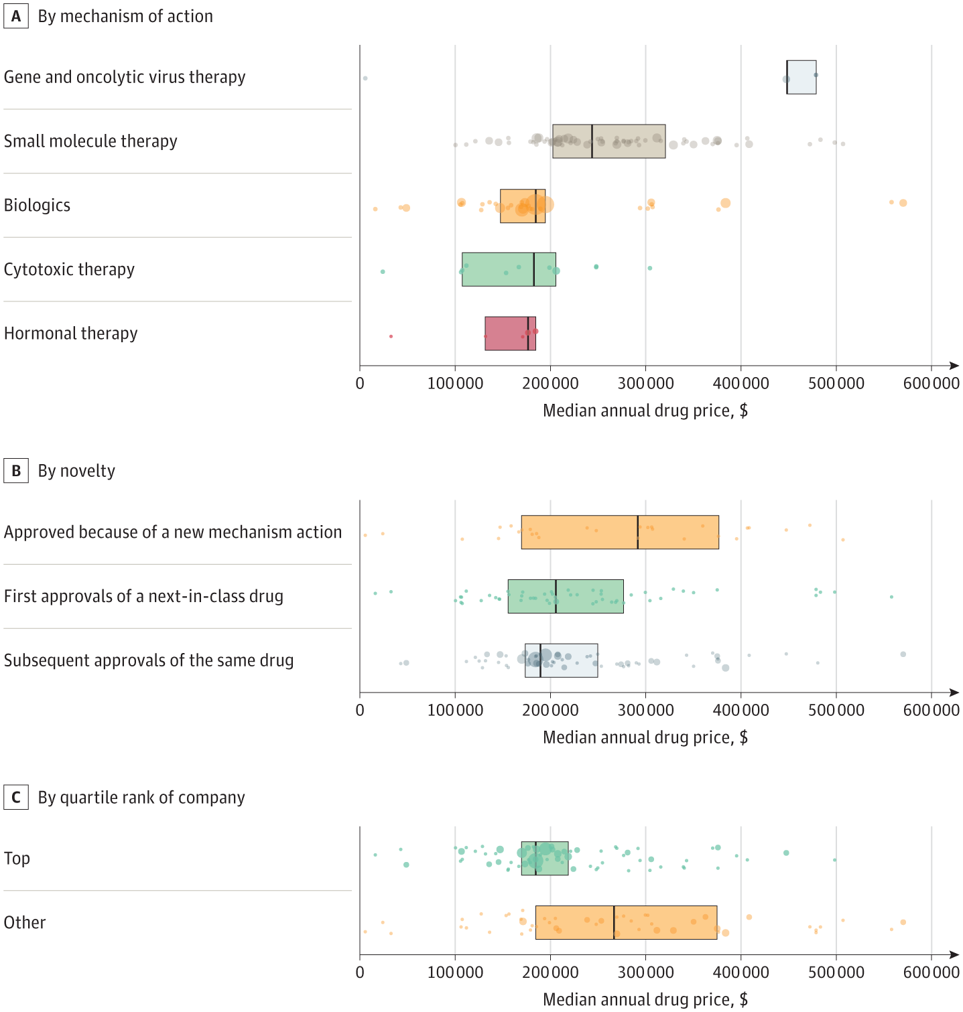
In a paper by Miljković et al. (2023), the authors find that the answer to the question, “Do first-in-class cancer drugs receive a pricing premium?” is ‘no‘. They examined oncology treatments with FDA approvals between 2015 and 2020 and identified average wholesale prices from Redbook. The drugs were categorized into three groups: (i) first approval of a new mechanism of action compound, (ii) next-in-class approval regardless of the tumor type, and (iii) subsequent approval of the same drug. Using this approach, they determined that:
There were 224 cancer drug approvals across 119 individual drugs, with a median annual cost of $196 000 (IQR, $170 000-$277 000). Gene and viral therapies were the most expensive (median, $448 000 [IQR, $448 000-$479 000]), followed by small molecule therapy (median, $244 000 [IQR, $203 000-$321 000), and biologics (median, $185 000 [IQR, $148 000-$195 000]). There was no significant difference in cost between first-in-class, next-in-class, and subsequent approvals of an already approved drug.
To access the full article, click here.
. If the provided articles seems to be less than 200 characters or it is an intro of the author, then try to generate an articles using this title. Do first-in-class cancer drugs receive a pricing premium? – Healthcare Economist




