Delays in drug approvals can result in significant financial losses for pharmaceutical companies. These costs include lost sales and increased expenses for prolonged clinical trials. Research by Smith, DiMasi, and Getz (2024) reveals that each day of delay can equate to around $500,000 in lost sales, with certain disease categories experiencing even higher daily sales losses.
The direct daily cost of conducting clinical trials is estimated to be about $40,000 for phase II and III trials, with certain therapeutic areas such as respiratory and rheumatology having higher daily costs.
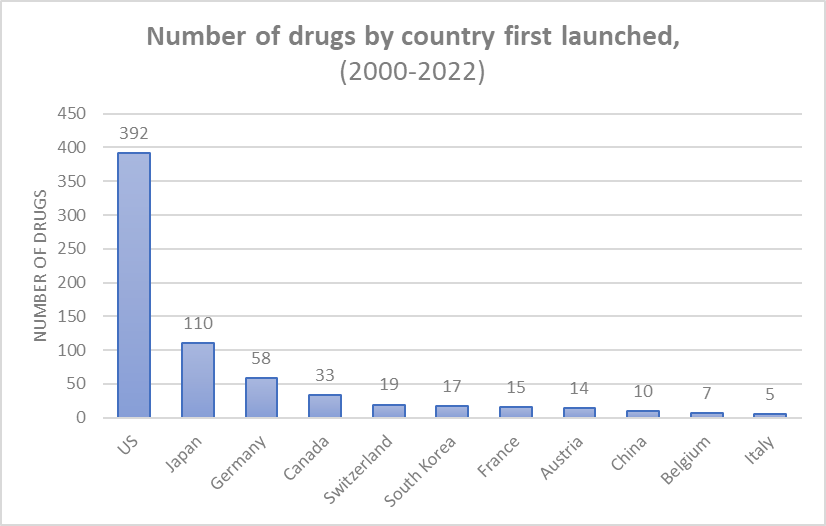
Approximately 60% of drugs are first launched in the US, with a significant percentage classified as “blockbuster” drugs generating over $1 million in daily sales. Cardiovascular, hematology, infection, and oncology drugs are among those with the highest daily sales figures.
On average, a clinical trial costs $25 million and takes over 600 days to complete. Phase III trials have the highest daily cost, followed by phase II, IV, and I trials.
The authors obtained drug revenue data from the Cortellis database and estimated drug development costs per day from Tufts University’s Center for the Study of Drug Development.