Samsung Bioepis has released a comprehensive Biosimilar Market Report for the second quarter of 2024. Here are some key highlights:
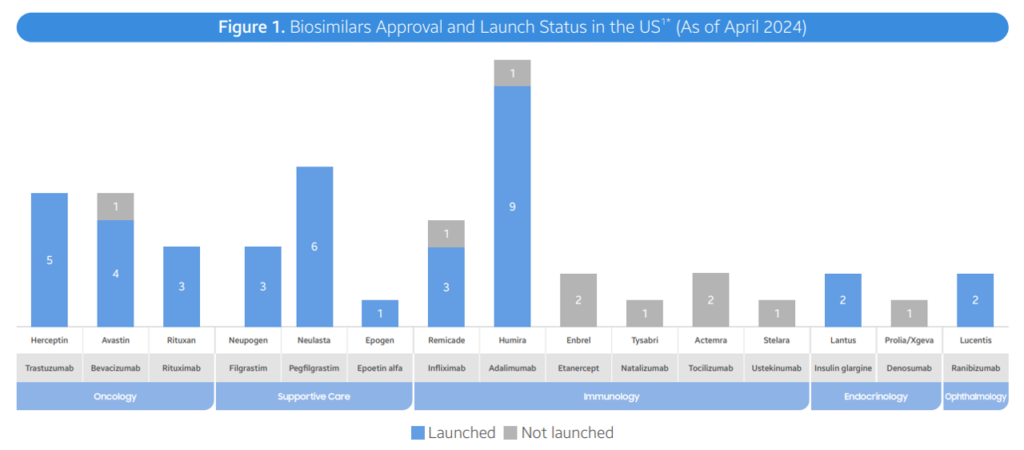
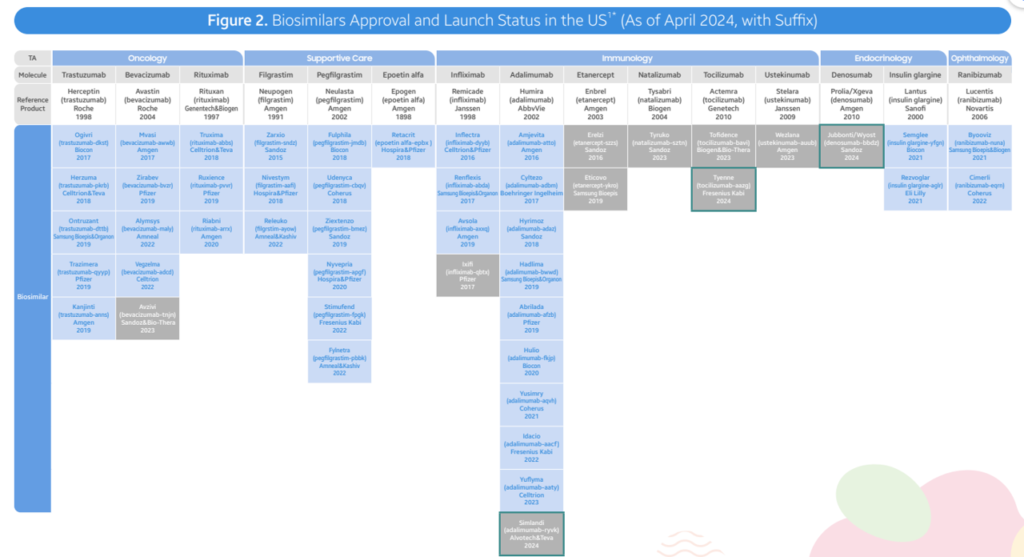
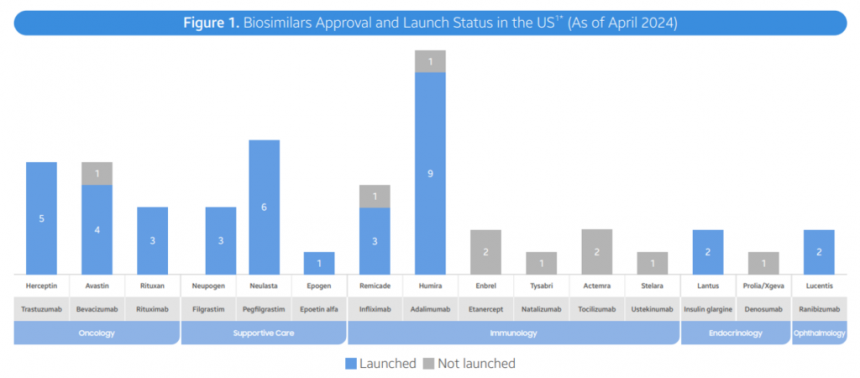
- FDA Approvals: As of April 2024, the FDA has approved a total of 48 biosimilars across 15 unique biological molecules. 38 of these biosimilars have already launched in the US market. In Q2 2024, 3 new biosimilars were approved, including Simlandi for Humira, Jubbonti/Wyost for Prolia/Xgeva, and Tyenne for Actemra.
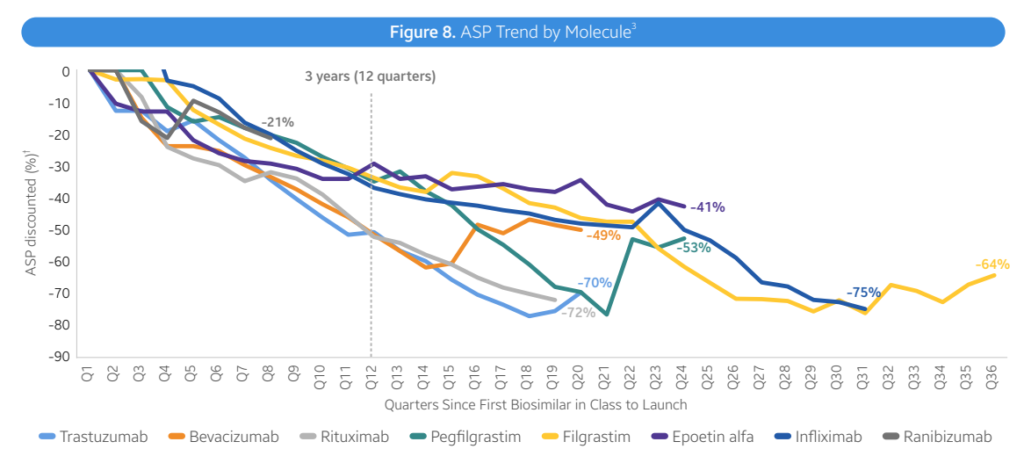
- Pricing Trends: Biosimilars have led to moderate discounts, with prices falling by 41% within 3 years. There is variability in price reductions across different therapeutic areas such as oncology, supportive care, immunology (Infliximab and Humira), ophthalmology, and diabetes.
- Biosimilar Uptake: The uptake of biosimilars varies by molecule, with higher market shares seen for drugs like bevacizumab and rituximab compared to insulin glargine and infliximab.
- Inflation Reduction Act: The act has both positive and negative implications for biosimilars. While some provisions may lead to higher list prices, others may impose pricing pressure on certain drugs and reduce the savings offered by biosimilars to plans.
You can find the detailed report here.