Efficient access to life-saving medications is crucial for patients with serious illnesses. The European Medicines Agency (EMA) plays a key role in expediting drug approvals to ensure safety and effectiveness. According to Grünwald and Stargardt (2024), the EMA was established in 1995 to harmonize pharmaceutical marketing authorization in the EU and EEA due to discrepancies in launch delays and availability of drugs across European countries.
The EMA implements three main community procedures to facilitate market access in EU member countries:
- Centralized procedure (CP): Ensures binding marketing authorization for all EU member states upon approval by the EMA. Originally used for biotechnological processes, it now includes various treatments like orphan drugs and substances for cancer, diabetes, HIV/AIDS, and other diseases.
- Mutual recognition procedure (MRP): Allows the applicant to choose a reference member state for evaluation, with subsequent adoption by all other member states seeking market access. This procedure covers pharmaceuticals outside the CP scope.
- Decentralized procedure (DCP): Permits manufacturers to seek country-specific approval for new substances not governed by CP or MRP.
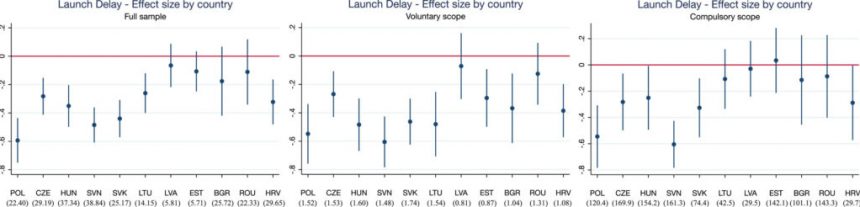
Grünwald and Stargardt (2024) conducted a study comparing countries subject to these procedures in the EU against non-EU countries to examine launch delays and availability of new active substances. IQVIA sales data from 33 European countries highlighted a mean decrease in launch delay of 10.9 months post-EU accession. The authors noted that strategic decisions by manufacturers at the national level may impact the effects of centralized marketing authorization on launch delay.
For further insights, refer to the complete article here.