Utilization management, such as prior authorizations and step edits, are often associated with higher cost branded products and biologics. Generic drugs, on the other hand, generally have low cost sharing and limited utilization management. However, the question of whether payers’ utilization management practices for biosimilars resemble those of biologic products or small-molecule generics, or fall somewhere in between, remains.
A study by Yu et al. (2023) seeks to address this question. The authors analyzed data from the Tufts Medical Center Specialty Drug Evidence and Coverage (SPEC) database, which covered 19 commercially-available biosimilars corresponding to 7 reference products, used for 28 unique indications. The study found that:
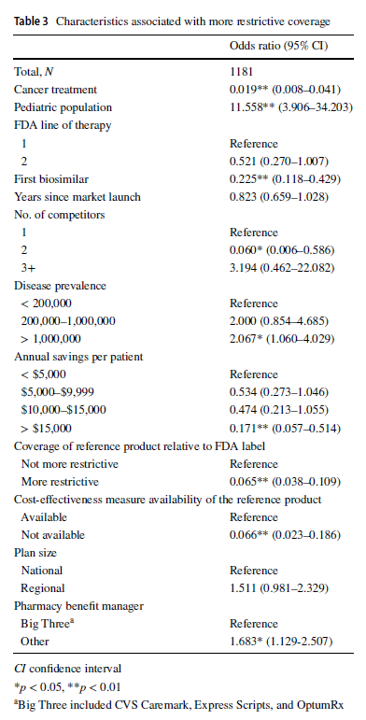
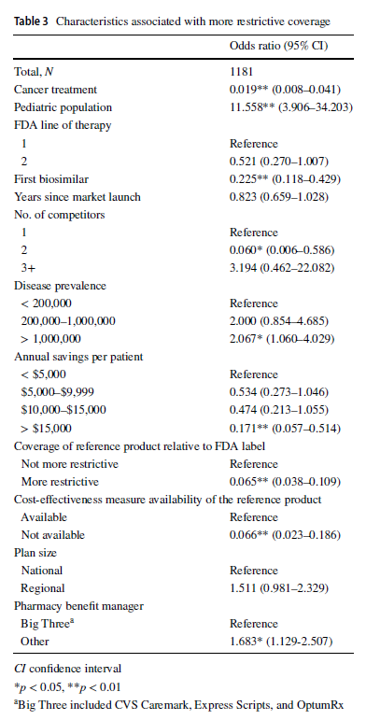
Compared with reference products, health plans imposed coverage exclusions or step therapy restrictions on biosimilars in 229 (19.4%) decisions. Plans were more likely to restrict biosimilar coverage for the pediatric population, in diseases with US prevalence higher than 1,000,000, and if the plan did not contract with one of the three major pharmacy benefit managers. Conversely, plans were less likely to impose restrictions on the biosimilar–indication pairs if the biosimilar was indicated for cancer treatments, if the product was the first biosimilar, if the biosimilar had two competitors, or if the biosimilar could generate annual list price savings of more than $15,000 per patient, among other factors.
One interesting finding was that large pharmacy benefit managers (PBMs) had less restrictive policies over biosimilars compared to smaller PBMs. The authors posited that the bargaining power of larger PBMs may lead to lower list prices and higher rebates, ultimately bringing more value to them.
You can read the full paper here.